Fecal incontinence affects 9 to 40% of hospitalized patients in the United States. Certain aspects of being in the hospital increase the risk of incontinence. Being on a ventilator, restricted to bed rest, taking antibiotics or other medications, or have a procedure or equipment that makes walking difficult can also contribute to fecal incontinence.
Fecal incontinence negatively affects quality of life and has negative effects on a person’s physical, emotional, and social health. Previous methods of controlling fecal incontinence have produced adequate results, but they also present a risk for complications. FI management in intensive or acute care settings is particularly challenging. FI management can take up to 170 mins per day, require multiple care providers, and can decrease the quality of life if mismanaged. Absorbent pads cause Incontinence associated dermatitis, cross contamination, and increase the likelihood of Hospital Acquired Complications such as CDI, HAPI etc. Balloon catheters may provide a closed stool management system but cause a myriad of complications due to high pressure balloon, such as – necrosis, mucosal injury, sphincter damage, bleeding and even perforation.
IBCs are inserted into the rectum and then inflated with air or water. The inflated balloon creates a sense of fullness that can help patients regain control over their bowel movements. While intrarectal balloon catheter have been shown to be effective for some patients, there have been reports of clinical complications associated with their use. Anchored at the anorectal junction, these high-pressure balloons can cause the patient discomfort, sphincter, and mucosal injuries. The user experience of intrarectal balloon catheters varies among individuals.
The US Food & Drug Administration maintains MAUDE (Manufacturer and User Facility Device Experience), that contains reports of adverse events, product problems, and medication errors associated with medical devices. The database is used by the FDA to monitor the safety and effectiveness of medical devices and to identify potential safety issues. There have been several MAUDE complaints related to intrarectal balloon catheter. One of the most common complications associated with the use of intrarectal balloon catheters is rectal bleeding. This can occur due to the trauma caused by the device’s insertion or removal, or due to over-inflation of the balloon. Over-inflation or high radial pressure of the balloon are some of the most common causes for a range of complications due to IBCs, including perforation of the rectum, leakage, patient discomfort, and many other problems. These complications can lead to significant morbidity and mortality, and therefore, it is essential to be aware of them.
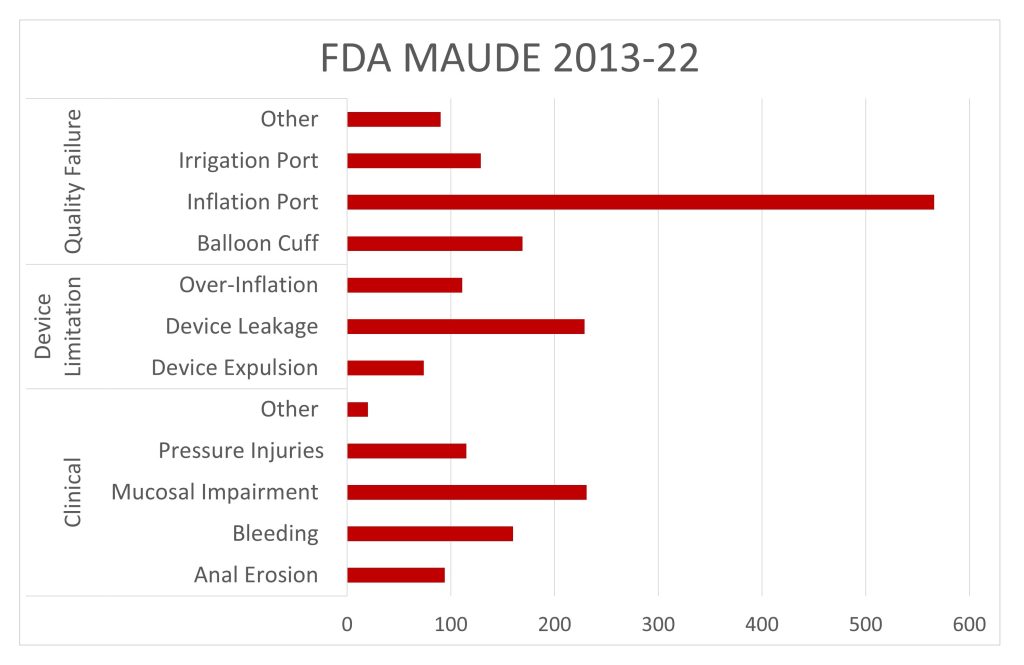
FDA MAUDE IBC’s 2013-2022
Qora – a non-balloon fecal management system
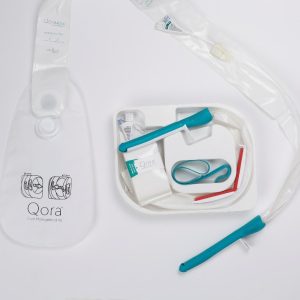
Qora Stool Management Kit
Qora is industry first non-balloon fecal management systems. Rather than employing a high-pressure balloon, this unique product uses a pliable lattice that moves with peristalsis. This unique feature allows the device to work in sync with the body, instead of against the patient’s physiology. Moving with peristalsis is also significantly less traumatic on the patient’s rectal mucosa.
Qora prevents sphincter damage that other fecal pouch may cause. Internal and external sphincters work with the surrounding pelvic muscles to create a barrier that prevents the escape of feces and help create continence. Trauma because of high pressure balloons could cause permanent damage to these muscles and cause minor to moderate incontinence.
Qora is also compatible with atonic sphincter, a condition that occurs when the anal sphincter lacks muscular tone.
Qora out-performs other approaches to fecal incontinence, and presents fewer risks. In fact, there are currently fewer than 5 reports about Qora on MAUDE. This makes Qora one of the best options for the management of fecal incontinence.
For more information on avoiding clinical complications and improving user experience for both patients and for nurses, consult with Consure Medical at [email protected].

One reply on “User Experience of Intrarectal Balloon Catheters and Clinical Complications Reported on FDA MAUDE”
Glue Dream strain I do not even understand how I ended up here, but I assumed this publish used to be great